PKD Outcomes Consortium (PKDOC)
2021 PKD Regulatory Summit

The Critical Path Institute’s Polycystic Kidney Disease Outcomes Consortium (PKDOC), the PKD Foundation, and Otsuka collaborated to create the 2021 PKD Regulatory Summit on May 19–20, 2021. Together with key stakeholders from the pharmaceutical industry and academic setting, foundations, patient advocacy groups, individuals living with PKD and regulatory agencies from around the world, the consortium addressed current unmet drug development needs for PKD, identified tools that are needed to deliver new therapies to patients, and catalyzed the development of a regulatory framework to enable advancement of new treatments.
The Summit included a series of three sessions with pre-recorded webinars available for registrants to view at their own pace two weeks prior to the meeting. On May 19 and 20, live presentations summarized content from the pre-recorded webinars, followed by panel discussions with speakers to facilitate dialogue among all participants.
Watch the sessions
In August 2022, four reports summarizing Sessions 1, 2, and 3B from the 2021 PKD Regulatory Summit were published in the Clinical Journal of the American Society of Nephrology (CJASN).
The PKD Outcomes Consortium (PKDOC) is a significant collaboration between the PKD Foundation, Critical Path Institute, representatives of the pharmaceutical industry, PKD clinicians, and the U.S. Food and Drug Administration (FDA). It was created to facilitate clinical trial development for PKD therapies by establishing a clear regulatory pathway for the pharmaceutical industry to evaluate the effectiveness of potential treatments.
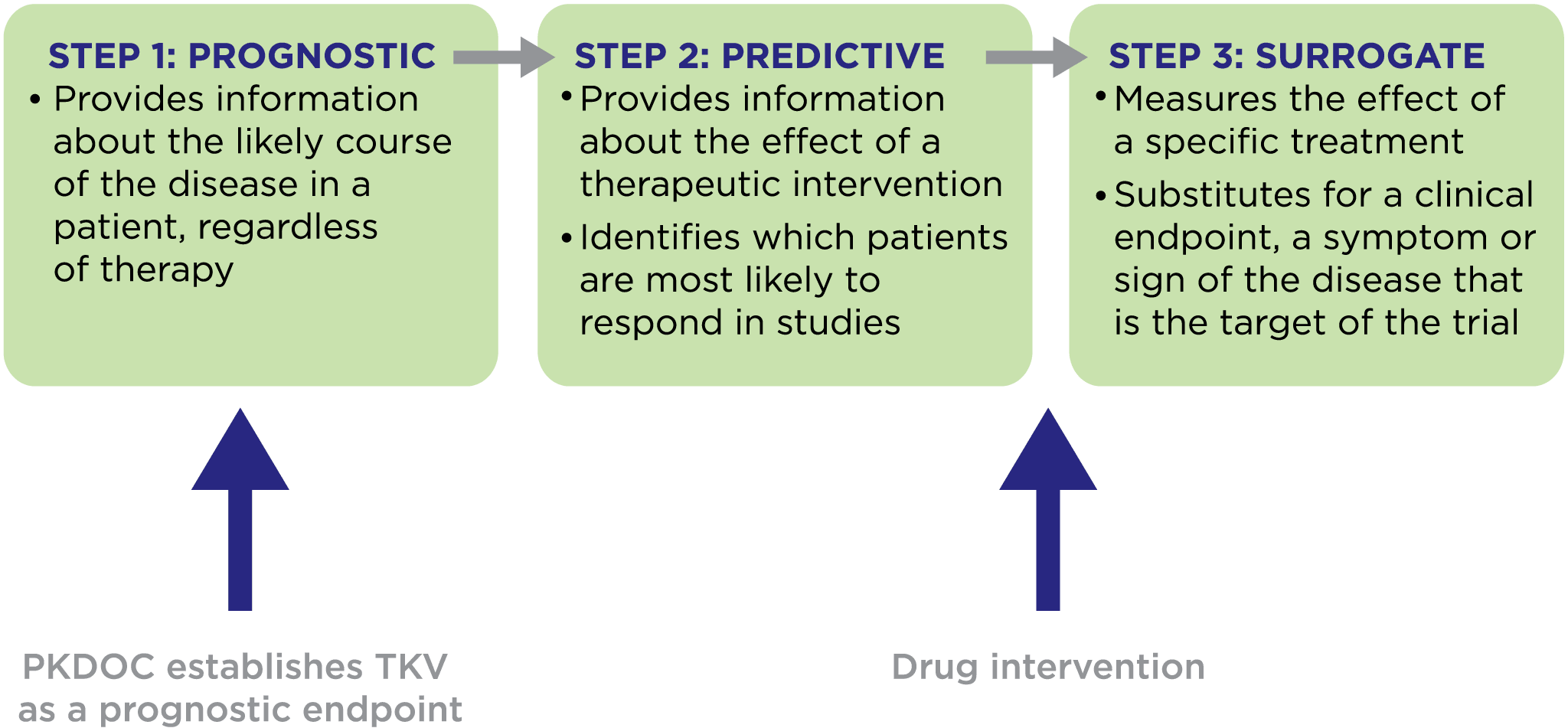
The consortium has successfully qualified Total Kidney Volume as a prognostic biomarker with both the US Food and Drug Administration and the European Medicines Agency. Currently, PKDOC hosts monthly teleconferences to explore alternative endpoints, innovative trial designs, and other topics of general interest to the participants. Attendees include pharmaceutical companies, academic organizations, foundations, patient advocacy groups, and regulatory agencies from around the world. Anyone who is interested may participate.
Why is PKDOC important?
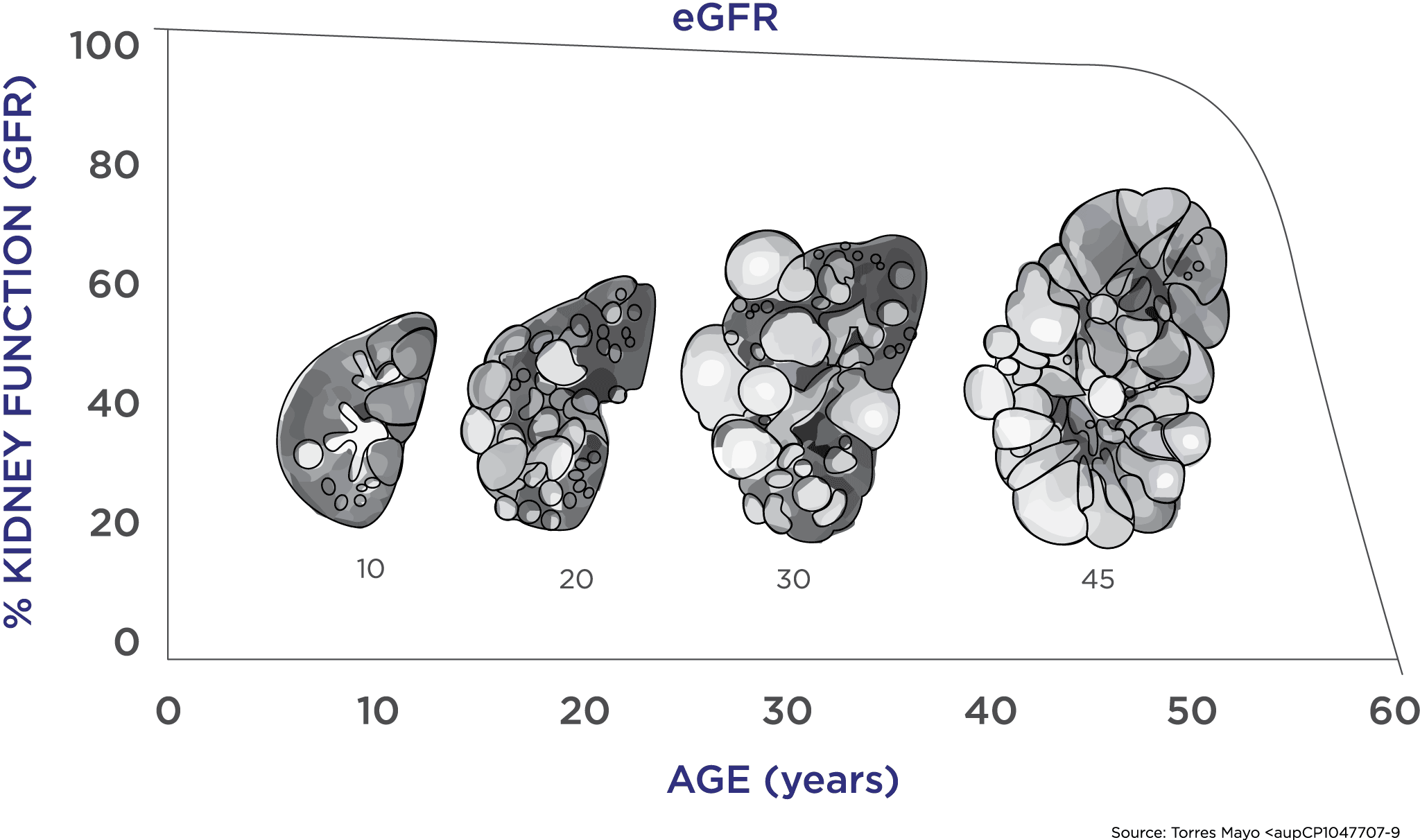
PKD is marked by a long period of stable kidney function (as measured by the currently accepted endpoints in drug development) during which the kidneys expand enormously due to cyst growth. Traditional endpoints of renal function (serum creatinine levels) only show changes very late in the course of the disease, making it difficult to assess the effectiveness of new medications. There is critical need for a biomarker that will assess disease progression at an earlier stage before patients have incurred serious, irreversible damage and when they may be more likely to respond to new therapies.
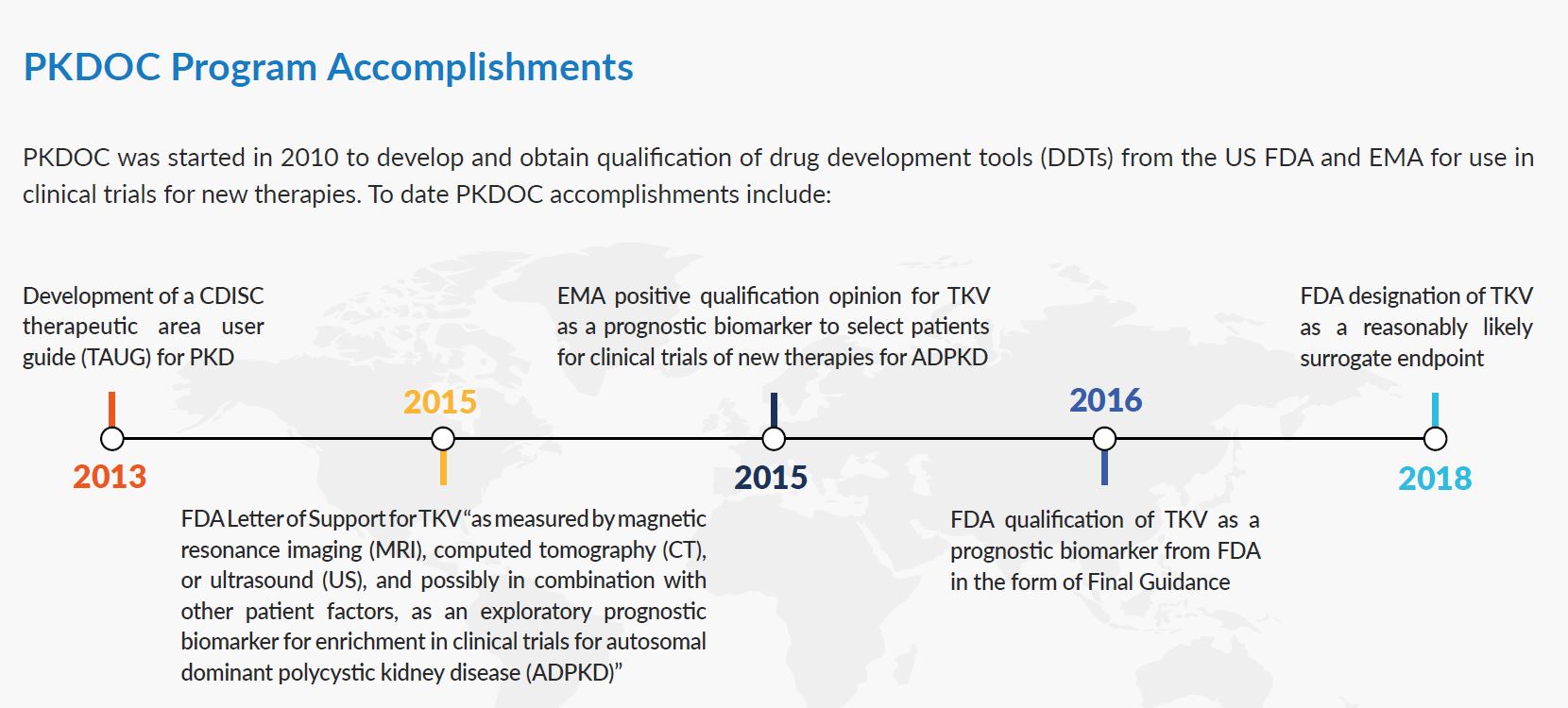
The initial goals of the PKD Consortium were to develop CDISC data standards for PKD and to use clinical data from ADPKD patients collected over many years in patient registries and observational studies to support the FDA and EMA qualification of an imaging biomarker, Total Kidney Volume (TKV), for use in drug development trials. These initial goals have been achieved.
Using the data collected, scientists were able to develop a disease progression model that evaluated the relationship between TKV and the known complications of ADPKD, including rate of loss of kidney function, hypertension, gross hematuria, kidney stones, urinary tract infections, development of end-stage renal disease, and mortality. These analyses were used to support the regulatory qualification of TKV as an accepted measure for assessing the progression of ADPKD in clinical trials in which new therapies are tested.
What is a prognostic endpoint?
Where has progress been made?
FDA Qualifies Total Kidney Volume as a Prognostic Biomarker for use in Clinical Trials for Polycystic Kidney Disease
On August 31, 2015, C-Path announced that the U.S. Food and Drug Administration (FDA) had issued a qualification decision in the form of a draft guidance to C-Path’s Polycystic Kidney Disease Outcomes Consortium (PKDOC) for total kidney volume (TKV) as a prognostic biomarker to select patients for clinical trials of new therapies for Autosomal Dominant Polycystic Kidney Disease (ADPKD).
- Details of the FDA Qualification Review
- FDA Guidance Document
- FDA Drug Development Tools Qualification Programs
EMA Renders Positive Qualification Decision for Total Kidney Volume as a Prognostic Biomarker for use in Clinical Trials for Polycystic Kidney Disease
On November 13, 2015, C-Path announced that the European Medicines Agency (EMA) rendered a positive qualification opinion to C-Path’s Polycystic Kidney Disease Outcomes Consortium for total kidney volume (TKV) as a prognostic biomarker to select patients for clinical trials of new therapies for Autosomal Dominant Polycystic Kidney Disease (ADPKD).
C-Path’s PKDOC and The PKD Foundation Set to Collaborate Once Again
On August 21, 2020, to meet the challenge in discovering treatments for PKD, C-Path’s Polycystic Kidney Disease Outcomes Consortium (PKDOC) announced a collaboration with the PKD Foundation, who’s mission is to fund research, advocate for patients and build a community for all impacted by PKD. The agreement renews the significant collaboration between C-Path and The PKD Foundation to facilitate existing and new mutually agreed upon programs to accelerate the pace and reduce the cost of medical product development for the diagnosis and treatment of PKD.


Get the latest information on treating PKD.
Page last updated November 2022









