 Published on January 26, 2021 | Staff at the PKD Foundation know that without patient stories, we couldn’t understand what it’s like to live with PKD. We’re always leaning on patient perspectives: when we talk with researchers about new studies, when advocating for resources at the federal level, and as we develop new educational tools for both patients and clinicians. The value of the patient experience is irreplaceable. That’s why collecting details of the disease experience from as many patients as possible is so important, and why we’re so excited about the ADPKD Registry.
Published on January 26, 2021 | Staff at the PKD Foundation know that without patient stories, we couldn’t understand what it’s like to live with PKD. We’re always leaning on patient perspectives: when we talk with researchers about new studies, when advocating for resources at the federal level, and as we develop new educational tools for both patients and clinicians. The value of the patient experience is irreplaceable. That’s why collecting details of the disease experience from as many patients as possible is so important, and why we’re so excited about the ADPKD Registry.
How does the ADPKD Registry work? In our first research poster, presented at the American Society of Nephrology’s Kidney Week in October, we outline the basics of participant enrollment and our methodology. 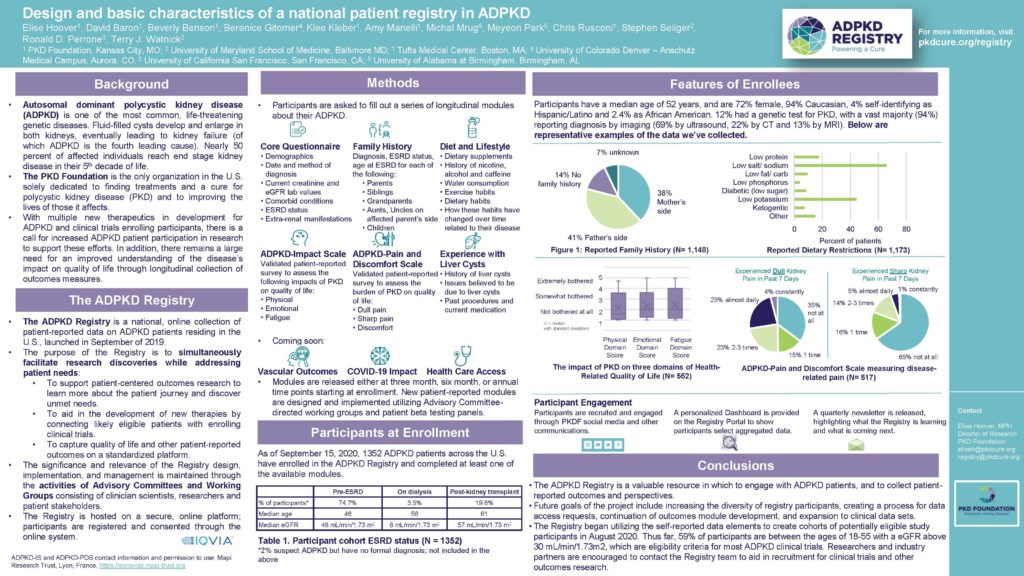
Learning from other Patient Registries
The Foundation isn’t just hoping the Registry will have an impact on the community. We believe that it will from what we’ve seen in other disease organizations.
The Cystic Fibrosis (CF) Foundation used its patient registry to observe trends in disease progression, medication effectiveness, and patient outcomes in order to create standards of care in CF clinics.
The National Registry of Rare Kidney Diseases in the United Kingdom have matched their participants to multiple studies and provided advice on trial design, eligibility criteria, and study site locations.
The Michael J. Fox (Parkinson’s) Foundation uses its Fox Insight Registry to collaborate with 23andMe and collect genetic data and increase access to a wider patient community.
The key to the success of these programs is their longitudinal data collection—participants don’t just sign on once. Many have been enrolled in the registries for years and continue providing data to update the organizations and researchers on their disease experience. By learning from the success of other organizations, the ADPKD Registry can grow into these kinds of initiatives too and accelerate progress toward our mission.
Growing the Registry
Beyond enabling the PKDF to collect data about the patient experience, the ADPKD Registry helps match individuals to clinical studies. The faster PKD studies enroll patients, the quicker the study will be complete and the FDA can evaluate the data for new potential therapies. Once Registry participants complete their modules, we let them know if they might be a good fit for a study. Enrollment in studies is based on age, disease stage, kidney function, and other factors. This data also helps drug developers know the size of the ADPKD population available for studies and their location throughout the U.S. to identify new trial sites. The Registry data shared with researchers and clinical study sponsors doesn’t include individual patient contact information or other identifying data. The Registry portal is hosted on a secure platform that follows strict guidelines to protect patient information. When an individual joins the Registry, they’re assigned a unique numeric identifier connected to module responses. This protects the confidentiality of all those who participate—the PKDF takes patient confidentiality and data security very seriously.
You Can Make a Difference
To help advance knowledge of disease progression and support the development of new treatments, we ask patients with ADPKD to join the ADPKD Registry. If you’ve already signed up, make sure to fill out your modules! Some are new, like our COVID-19 Impact module or the ADPKD-Impact Scale. Others, you’ll need to fill out again, like our Core Questionnaire. We ask you to update this every year so we know your latest kidney function, symptoms, and other factors about your PKD progression. As of today, nearly 2,000 ADPKD patients have signed on to the research program. Want to know more about what we’re learning? Sign up for our webinar where we’ll share results from the first ADPKD Registry Annual Report on March 23 at 7:00 p.m. (EST).









Is there an ARPKD registry?
Great question, Sarah! You can find a link to an ARPKD registry on our “What Is ARPKD?” page.