
Published on September 20, 2022 | Last year, the Critical Path Institute’s Polycystic Kidney Disease Outcomes Consortium (PKDOC), the PKD Foundation, and Otsuka hosted the 2021 PKD Regulatory Summit. In addition, key stakeholders from the pharmaceutical industry and academic settings, patient advocacy groups, PKD patients, families, and regulatory agencies from around the world attended. Collectively, they addressed the current unmet drug development needs for PKD, identified tools needed to deliver new therapies to patients, and catalyzed a path to get treatments to patients faster.
What is PKDOC?
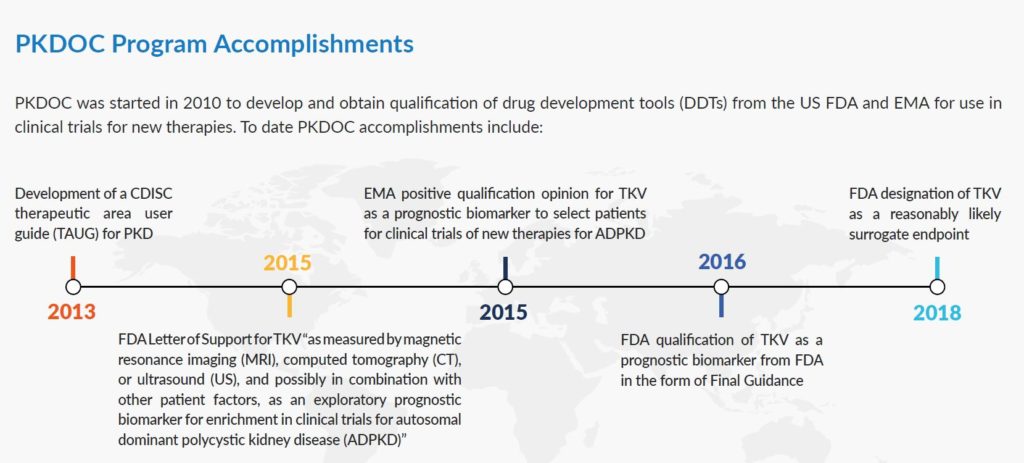
The PKD Outcomes Consortium (PKDOC) is a significant collaboration between the PKD Foundation, Critical Path Institute, representatives of the pharmaceutical industry, PKD clinicians, and the U.S. Food and Drug Administration (FDA). What does it do? It facilitates the development of PKD therapies by developing tools (eg. biomarkers) and resources (eg. databases) to accelerate the evaluation of the safety and effectiveness of potential treatments.
Initially, the PKDOC’s goals were, firstly, to develop data standards for PKD. Data standards simplify starting clinical studies and ensures information vital to evaluating a potential treatment is collected. And secondly, to use clinical data from ADPKD patients collected over many years in patient registries and observational studies to support the FDA and EMA qualification of total kidney volume (TKV) for use in drug development trials. We’re happy to report that those initial goals are complete.
2021 PKDOC Regulatory Summit Recap
That brings us back to the PKDOC Summit. Prior to the summer, a series of three sessions, with pre-recorded webinars, allowed registrants to prepare for discussions. At the summit, live presentations summarized content from those webinars, followed by panel discussions. This even included discussions with the FDA to increase the chances of research on both ARPKD and ADPKD to drive new treatments. It takes a unique organization like the PKD Foundation to facilitate so many experts in one place. Without it, these critical conversations are unlikely to happen.
Post-PKDOC Summit
Since the PKDOC Summit, four reports summarizing sessions 1, 2, and 3B have been published in The Clinical Journal of the American Society of Nephrology (CJASN). These article focus on drug development and clinical trial design.

“We’re hoping the findings outlined in these papers will frame the approach and development of ARPKD and ADPKD treatments for the next 5+ years.”
—Chris Rusconi, Ph.D., Interim CEO and Chief Research Officer
Drug Development in Cystic Kidney Disease
This article summarizes the PKDOC Summit and where treatments currently stand for ARPKD and ADPKD. Among its points, it highlights the need for continued collaboration and data-sharing efforts to develop new treatments.
“I’ll never be able to find the right words to express what it meant to me to be a part of that trial [tolvaptan]. Not only for the benefit I may have gotten, but for the countless patients waiting for a medication like it.”
—Cari Maxwell, PKDOC Summit Patient Speaker
Perspectives on Drug Development in ARPKD
When it comes to ARPKD clinical research and drug development, several things make it challenging. Firstly, the disease is rare so it’s hard to fill up clinical trials with patients. Secondly, limited preclinical models make it hard to study even in animals. Thirdly, knowledge gaps on the progression of PKD. Since the PKD journey varies person to person, it’s hard to pin down biomarkers and measure disease progression. And finally, a lack of clearly defined applicable primary end points for clinical trials. In this article, experts outline those challenges in more detail as well as three next steps:
-
- Develop large, international observational studies
- Identify prognostic biomarkers (markers of disease progression) to use in clinical trials
- Test out potential treatments
Perspectives on Drug Development in Early ADPKD
In this paper, experts discuss how current therapies (tolvaptan and those in the pipeline) are restricted to adults at risk of rapid progression. Among the paper’s contributors are Scientific Advisory Panel member, Neera Dahl, M.D., and ADPKD patient, Hayley Womack. They outlined how tolvaptan is indicated to slow kidney function decline in adults at risk of rapidly progressing ADPKD. But what about its effectiveness on younger patients with ADPKD?
“Everyone with ARPKD has a really different progression, and we still don’t know what to expect most of the time.”
—Julie Marshall, ARPKD Parent and Board of Directors Member
Data on tolvaptan in children with ADPKD should be available soon. To explore it as a possible treatment option, authors explained future research steps.
- Determine better biomarkers of progression
- Include patients with slower progression or young adults in trials
- Add patient-reported outcomes to trials (e.g. pain or quality of life)
- Look at issues outside of the kidneys in clinical trials (e.g. effects on the liver)
Current Challenges and Perspectives on Developing a Clinical Trial Design for ADPKD
What would an ideal clinical trial protocol in ADPKD look like? This article proposes one possible perspective.
Through a workshop, key stakeholders established near-term and longer-term goals for the ADPKD research community to consider. Together, they identified a major goal—the need to standardize key elements of clinical trial protocols necessary to support a common approach to ADPKD clinic trials. In order to facilitate and potentially accelerate the development of therapies, a common trial approach could including:
- Standardizing how we collect key elements to be able to combine results
- Designing trials that are accessible to patients to enter and remain for long durations
- Leveraging technology to promote remote patient visits and monitoring
“I truly applaud the work being done at this conference to create more efficient, meaningful clinical study designs. This collaboration between stakeholders allows for easier and consistent data. Thus, clearer study outcomes.”
—Greg Mainolfi, ADPKD Patient
As a result of conversations at the 2021 PKDOC Summit, it’s clear the field of drug treatments for PKD is bright. With the perspectives outlined in these four articles, it confirms the value of collaboration between the wide range of PKD stakeholders. When it comes to ending PKD, it takes all of us working together.









Has this foundation ever researched Santa Barbara Nutrients and the effects keto citra has on polycystic kidney disease? My cousin has been taking this supplement and eliminating certain foods from his diet for several months. His kidney function has improved 10 points.
Hi Susan, it is recommended to discuss any changes to your diet and lifestyle with your nephrologist to ensure they are safe for you. A first clinical trial from one of our grantees has shown that a ketogenic diet is feasible in patients with cystic kidneys under the guidance of a kidney specialist, although the researchers want to obtain more data regarding safety. You can read more at https://t.co/YgcOkw3C7I
Do you have dietary recommendation for our 11.5 year old cat with PKD. His numbers suddenly worsened to early Stage 3 from Stage 1. We started Ketro-Citra and after a month his SDMA went from 16 to 11.4 but Creatine and Urea nitrogen remained elevated. The science diet kidney foods have carrots and we have to take many tiny carrots from each serving before giving him the soft food. We are also using Royal Canin kidney dry food. We are administering 1mg lactated ringers subcutaneously every day for him. Thank you.
Hi Susan. Our organization is solely focused on polycystic kidney disease in humans.