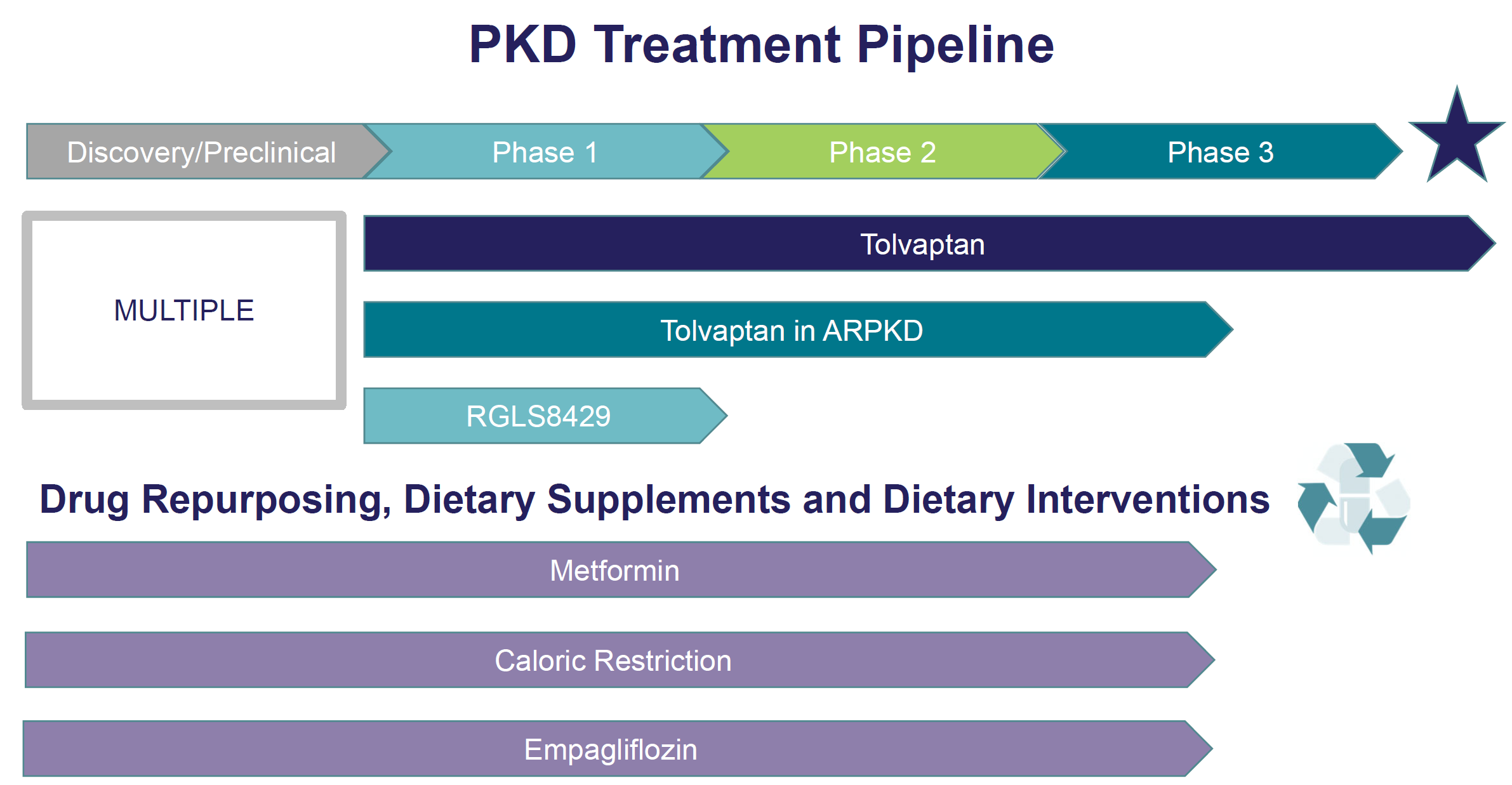
Research pipeline
Since 1982, we’ve led the fight against PKD through the support of basic, translational, and clinical scientists; vital research funding; and patient education. Today, we’re encouraged by the significant strides we’re making to find treatments. We’ve gone from a single drug in clinical trials five years ago to an approved drug, tolvaptan, and more drugs in the pipeline today than ever before.
Read on to learn more about the treatments for PKD currently being developed in the United States.
Learn more about clinical trials and the difference between Phases 1–3.
Patients play a key role in the research and development process by volunteering to participate in clinical studies. From observational studies to clinical trials, you can help researchers unlock the secrets of PKD and find a treatment by participating in a study. Learn more.
Receive notifications when there are clinical studies in your area.
PKDF Research Programs
Additional Resources
- Dialysis 101
- Managing nutrition as dietary needs change from pre-dialysis to post transplant
 Tolvaptan
Tolvaptan
On April 24, 2018, the U.S. Food and Drug Administration (FDA) granted approval of tolvaptan to be the first treatment in the United States for adult patients with autosomal dominant polycystic kidney disease (ADPKD), the most common form of polycystic kidney disease (PKD).
Tolvaptan is a medication (taken twice a day as an oral pill) that affects how the kidneys control the concentration of urine. It’s been shown to slow down the growth of kidney cysts (total kidney volume) when it is taken for a long time (several years) by adults at risk of rapidly progressing ADPKD. This may help protect the function of your kidneys and delay the need for a kidney transplant or dialysis. Though your kidney function would continue to decline, it would be at a slower rate. Learn more here.
 Tolvaptan in ARPKD
Tolvaptan in ARPKD
What it is
Tolvaptan was approved by the FDA to treat ADPKD in 2018. It is a medication (taken twice a day as an oral pill) that affects how the kidneys control the concentration of urine. It’s been shown to slow down the growth of kidney cysts (total kidney volume) when it is taken for a long time (several years) by adults at risk of rapidly progressing ADPKD. It is now being studied in pediatric ARPKD patients.
Why it may be a potential treatment for PKD
Tolvaptan is an approved drug that inhibits the vasopressin pathway by blocking the activity of the vasopressin receptor 2 (V2). Preclinical studies of tolvaptan in rat models of PKD in which the causative gene of ARPKD, PKHD1, is mutated, demonstrated a reduction in kidney cyst formation, decreased cyst cell growth, and preserved kidney function, suggesting the vasopressin pathway may also play a role in the progression of the renal manifestations of ARPKD. The potential role of the vasopressin pathway in promoting cyst formation caused by mutations in PKHD1 has also been confirmed by genetic studies in rat.
Clinical study status
Otsuka is currently studying tolvaptan in a two phase 3 studies in ARPKD. These are an 18 month and a 2-year study, each evaluating the safety and efficacy of tolvaptan in up to 20 patients under the age of 18. Read more to learn about what participating looks like.
 RGLS4326/ RGLS8429
RGLS4326/ RGLS8429
What it is
RGLS4326 is a novel drug candidate that belongs to a class of drugs that target microRNAs. Micro RNAs are a collection of RNA molecules in cells that play multiple roles in the regulation of cell function. RGLS4326 is being developed by Regulus Therapeutics for ADPKD.
Why it may be a potential treatment for PKD
The specific microRNA targeted by RGLS4326, miR-17, is involved in regulating the production of PKD1 and PKD2 proteins, the proteins encoded by the causative genes in ADPKD. Preclinical studies with RGLS4326 have demonstrated direct regulation (an increase in the amounts produced) of PKD1 and PKD2 in human ADPKD cyst cells, as well as a reduction in kidney cyst formation, decreased cyst cell growth, and preserved kidney function in animal models of ADPKD. A phase 1 multiple dose study of RGLS4326 was completed in healthy volunteers, and demonstrated that the drug was generally well tolerated.
Clinical study status
Regulus first studied RGLS4326 for the treatment of ADPKD in a phase 1 dose ranging study in 27 patients in the U.S.. In late 2020, the company decided to stop this study to instead invest in another compound, stating “we have determined that advancing our next-generation compound RGLS8429 is more compelling than further development of RGLS4326.”
The RGLS8429 will begin recruiting in Fall 2022. We rely on ADPKD patients to sign up for the study to move this research forward. Learn more about what participating in PKD studies looks like here.
 Drug Repurposing
Drug Repurposing
Caloric Restriction
What it is
Daily caloric restriction (reducing total calories eaten per day) and intermittent fasting (limiting eating to only certain times of day) are two forms of dietary interventions for weight loss.
Why it may be a potential treatment for PKD
There’s a growing body of evidence that supports metabolic dysregulation (e.g. abnormal sugar use and storage or abnormal fat metabolism to generate energy within cells) helps drive ADPKD progression. Data from animal studies suggest that a variety of dietary interventions can slow disease progression in animal models of ADPKD. These dietary interventions point to several potential mechanisms by which dietary intervention slows ADPKD progression in animals—weight loss, caloric restriction, and/or periods of fasting—dependent or independent of metabolic reprogramming.
Clinical study status
There are multiple studies ongoing to better understand impact and safety of these diets on individuals with ADPKD. Find out what participation looks like here.
Curcumin
What it is
Curcumin is a dietary supplement that is produced by some plants and is found in the spice turmeric.
Why it may be a potential treatment for PKD
Curcumin activates transcription of key antioxidants, suppresses inflammation, and reduces cell proliferation (growth). Because of these properties, it’s thought that it could have positive effect in reducing cell growth as well as improve the health and function of arteries in ADPKD.
Clinical study status
The University of Colorado Anschutz Medical Campus recently finished a study to determine if curcumin can improve the function of blood vessels in children and young adults with ADPKD. In February 2022, investigators published their findings and unfortunately could find no benefit from curcumin supplementation on vascular function or kidney growth. Read more here.
Empagliflozin
What it is
Empaglifozin is an FDA-approved medication used to manage and treat type 2 diabetes.
Why it may be a potential treatment for PKD
Limited data suggests SGLT2i drugs (such as empagliflozin) may stimulate vasopressin and vasopressin receptor expression in patients and animal models without ADPKD. Investigators want to see if the beneficial effects on kidney function, vascular function, and mortality in non-ADPKD patients with CKD will translate to this disease population.
Clinical study status
The University of Colorado Anschutz Medical Campus and the University of Maryland Baltimore are conducting a study on empagliflozin. Learn about what participation looks like here.
Metformin
What it is
Metformin is a first-line, FDA-approved drug to treat type 2 diabetes.
Why it may be a potential treatment for ADPKD
Mouse models have shown that metformin blocks the aerobic glycolysis pathway, which is linked to cell proliferation that causes cysts to form and grow. When treated with metformin, mice with PKD show inhibition of cyst growth. Metformin has a long established safety profile because it’s been used for decades in the treatment of type 2 diabetes.
Watch our recorded webinar, PKD therapies and potential candidates, to learn more.
Clinical study status
Researchers at Tufts University in Boston and at the University of Maryland in Baltimore recently finished a two-year clinical trial in 97 patients with ADPKD. They found that metformin in adults with ADPKD was safe and tolerable while slightly reducing kidney function decline (although not to a significant degree). Further evaluation of efficacy will require a larger trial. Read more here.
Niacinamide
What it is
Niacinamide is a form of vitamin B3 available over the counter. It’s used for treating diabetes and certain skin conditions.
Why it may be a potential treatment for ADPKD
Niacinamide has been shown to inhibit a regulatory protein called sirtuin 1, which promotes cell growth and may promote cyst growth in PKD. Inhibition of sirtuin 1 may slow or stop cyst growth.
Niacinamide could be an appealing treatment option for PKD because of its low cost and favorable safety profile. The European Nicotinamide Diabetes Intervention Trial (ENDIT) study showed that large doses of niacinamide are safe in humans. Additionally, niacinamide is a dietary supplement, so it doesn’t require approval by the FDA. Studies aimed to establish an optimal dosage for ADPKD patients.
Clinical study status
The Randomized, Controlled Pilot Study of Niacinamide in Polycystic Kidney Disease (NIAC-PKD2), conducted at the University of Kansas Medical Center, was designed to determine the effects of niacinamide on markers of kidney injury, inflammation, kidney function, and cyst growth. Unfortunately, although the drug was well-tolerated in ADPKD patients, the researchers observed no benefit compared to those on placebo.
Pioglitazone
What it is
Pioglitazone is an FDA-approved treatment for type 2 diabetes.
Why it may be a potential treatment for ADPKD
Pioglitazone has been shown to inhibit chloride secretion into cysts, which is a key factor in cyst growth. When chloride moves into the cyst, water follows, filling the cysts with fluid and causing them to grow. By inhibiting chloride from moving into cysts, pioglitazone may potentially slow or stop cyst growth. Pioglitazone may also inhibit cyst cell division by causing terminal cell differentiation.
Watch our recorded webinar, A discussion about pioglitazone Actos as a potential therapy for PKD, to learn more.
Clinical study status
Researchers at Indiana University Purdue University in Indianapolis (IUPUI) conducted a pilot clinical trial to test the safety and efficacy of low doses of pioglitazone on ADPKD progression. In the 18 patients who participated in the study, no change in total kidney volume was observed over 1 year for those on study drug compared to placebo. However, pioglitazone was found to be safe in ADPKD patients, and a larger and longer-term trial would be needed to determine the effectiveness of the study drug to slow disease progression. Read more here.
Statin therapy
What it is
Statins comprise a class of drug that lowers the level of cholesterol in the blood.
Why it may be a potential treatment for PKD
In addition to their efficacy in lowering cholesterol, statins also have anti-proliferative, anti-inflammatory, and antioxidant effects. Due to this and their relative safety, it’s thought they could potentially be used to slow the progression of ADPKD.
Clinical study status
The University of Colorado Anschutz Medical Campus is conducting a study to learn if pravastatin (a common statin) is helpful in slowing down the progression of ADPKD. This study is no longer recruiting patients and results are expected to be available in late 2022.
Page last updated June 2023








