
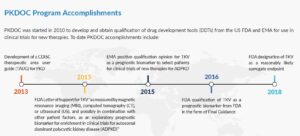
The consortium has successfully qualified Total Kidney Volume as a prognostic biomarker with both the US Food and Drug Administration and the European Medicines Agency. Currently, PKDOC hosts monthly teleconferences to explore alternative endpoints, innovative trial designs for both ADPKD and ARPKD, new biomarkers, develop a PKD clinical trial simulator, and discuss other topics of general interest to the participants.
What has PKDOC done recently?
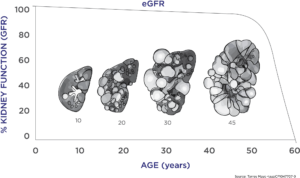
PKD is marked by a long period of stable kidney function (as measured by the currently accepted endpoints in drug development) during which the kidneys expand enormously due to cyst growth. Traditional endpoints of renal function (serum creatinine levels) only show changes very late in the course of the disease, making it difficult to assess the effectiveness of new medications. There was a critical need for a biomarker that would assess disease progression at an earlier stage before patients incurred serious, irreversible damage and when they would be more likely to respond to new therapies.
The initial goals of the PKD Consortium were to develop Clinical Data Interchange Standards Consortium (CDISC) data standards for PKD and to use clinical data from ADPKD patients collected over many years in patient registries and observational studies to support the FDA and European Medicines Agency (EMA) qualification of an imaging biomarker, Total Kidney Volume (TKV), for use in drug development trials. These initial goals have been achieved.
Using the data collected, scientists were able to develop a disease progression model that evaluated the relationship between TKV and the known complications of ADPKD, including rate of loss of kidney function, hypertension, gross hematuria, kidney stones, urinary tract infections, development of end-stage renal disease, and mortality. These analyses were used to support the regulatory qualification of TKV as an accepted measure for assessing the progression of ADPKD in clinical trials in which new therapies are tested. This was critical in getting approval for tolvaptan, the only FDA approved therapy for ADPKD.
What is a prognostic endpoint?
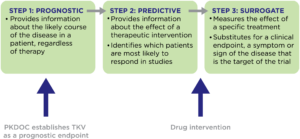
Where has progress been made?
Voice of the Patient report: Externally-Led Patient-Focused Drug Development (EL-PFDD) Meeting for ARPKD Patients and their Families
The Autosomal Recessive Polycystic Kidney Disease (ARPKD) Externally-Led Patient Focused Drug Development (EL-PFDD) was held virtually on August 29, 2023. The meeting was an important opportunity for the Polycystic Kidney Disease Foundation (PKDF) to share patient perspectives regarding the symptoms and daily impact of ARPKD, as well as current and future approaches to therapies. The virtual meeting format allowed many ARPKD community members to participate through live online polls, telephone call-ins, and by providing written comments through an online portal.
The final reports of the EL-PFDD meeting, the video recording of the meeting are available on the PKD Foundation website at ARPKD PFDD 2023.
Autosomal Recessive Polycystic Kidney Disease (ARPKD)Adjunct EL-PFDD Scientific Workshop Report
ARPKD Adjunct EL-PFDD Scientific Workshop, held on January 23, 2024, was a critical follow up to the EL-PFDD meeting. This workshop gave ARPKD clinical and research experts an opportunity to reflect on what was shared by ARPKD patients and families, and to identify important scientific priorities and data needed to move the field forward.
A video recording of the January 23, 2024 ARPKD Adjunct Scientific Workshop is available here.







 The Critical Path Institute’s Polycystic Kidney Disease Outcomes Consortium (PKDOC), the PKD Foundation, and Otsuka collaborated to create the 2021 PKD Regulatory Summit on May 19–20, 2021. Together with key stakeholders from the pharmaceutical industry and academic setting, foundations, patient advocacy groups, individuals living with PKD and regulatory agencies from around the world, the consortium addressed current unmet drug development needs for PKD, identified tools that are needed to deliver new therapies to patients, and catalyzed the development of a regulatory framework to enable advancement of new treatments.
The Summit included a series of three sessions with pre-recorded webinars available for registrants to view at their own pace two weeks prior to the meeting. On May 19 and 20, live presentations summarized content from the pre-recorded webinars, followed by panel discussions with speakers to facilitate dialogue among all participants.
The Critical Path Institute’s Polycystic Kidney Disease Outcomes Consortium (PKDOC), the PKD Foundation, and Otsuka collaborated to create the 2021 PKD Regulatory Summit on May 19–20, 2021. Together with key stakeholders from the pharmaceutical industry and academic setting, foundations, patient advocacy groups, individuals living with PKD and regulatory agencies from around the world, the consortium addressed current unmet drug development needs for PKD, identified tools that are needed to deliver new therapies to patients, and catalyzed the development of a regulatory framework to enable advancement of new treatments.
The Summit included a series of three sessions with pre-recorded webinars available for registrants to view at their own pace two weeks prior to the meeting. On May 19 and 20, live presentations summarized content from the pre-recorded webinars, followed by panel discussions with speakers to facilitate dialogue among all participants.