Throughout September, PKD Foundation leadership joined experts, advocates, academics, and pharmaceutical companies in Washington, D.C., for a variety of meetings to discuss PKD research. Together, they discussed everything from the state of federal research funding to strategies for accelerating PKD clinical trials.
Critical Path Institute Global Impact Conference
As our partner in advancing polycystic kidney disease (PKD) research, including PKD clinical trials, the Critical Path Institute (C-Path) Global Impact Conference brought together key stakeholders, ranging from academics to pharmaceutical companies.

PKD Foundation leadership and expert clinicians attend the Critical Path Institute Global Impact Conference. Left to Right: Mayo Clinic, Rochester Director Neera Dahl, M.D., PKD Foundation VP of Research Projects Chris Chen, Ph.D, PKDOC Director Sorin Fedeles Ph.D., PKD Foundation President and CEO Susan Bushnell, and PKD Foundation Chief Growth Officer Craig Robertson.
PKD Foundation Collaboration with C-Path
When it comes to healthcare, C-Path believes “it takes too much time and costs too much money to take a potential new medicine discovered in the laboratory through the drug development process and achieve a regulatory-approved safe and effective product.” To speed up this process, they bring together leaders from science, medicine, government, and patient advocacy. This not only helps advance the development of new treatments but also ensures patients’ voices are heard.
In 2010, the PKD Foundation co-founded the PKD Outcomes Consortium (PKDOC) as a program within C-Path. This partnership brings together representatives from the pharmaceutical industry, PKD clinicians, and the U.S. Food and Drug Administration (FDA). Together, it establishes a clear, official path for pharmaceutical companies to evaluate the effectiveness of potential treatments, facilitating the creation of clinical trials for PKD therapies.
How the C-Path Conference Supports PKD Clinical Trials
Altogether, the Global Impact Conference hosted more than 300 attendees. This unique opportunity connected PKD Foundation staff, clinicians, and researchers with the FDA and members of industry.
One event panel discussed “Reasonably Likely Surrogate to Accelerated Approval: Exploring PKD and Alzheimer’s Disease Approval Pathways.” This session included two PKD clinicians, both of whom have received PKD Foundation research grants: PKDOC Director Sorin Fedeles Ph.D., and Mayo Clinic, Rochester Director Neera Dahl, M.D.
They highlighted how their work in establishing total kidney volume (TKV) as an FDA-approved measurement tool for disease progression in PKD could be translated to Alzheimer’s Disease.
TKV and PKD Clinical Trials
Total kidney volume, or TKV, is a measurement of the volume of both kidneys. In PKD, cysts grow on the kidneys, increasing the volume. This measurement is critical for doctors to assess where patients are in their PKD journey and what’s possible.
Based on the size of a patient’s kidneys (as well as their age and height), doctors can say how aggressive the PKD is. By using that score, they can make predictions and outline what treatment options may be available to the patient.
Because of this milestone, tolvaptan was developed, the first treatment for PKD.
National Institute of Diabetes and Digestive and Kidney Diseases Meeting

When it comes to PKD research, federal funding is critical to progress. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institute of Health (NIH), supports research on chronic kidney conditions.
Recently, the PKD Foundation’s vice president of research programs, Chris Chen, Ph.D., met with the NIDDK’s chief of renal diagnostics and therapeutics, Robert Star, M.D., and program directors, Susan Mendley, M.D., and Christine Maric-Bilkan, Ph.D. Together, they discussed PKD Foundation priorities, NIH funding challenges, and the PKD Research Resource Consortium (RRC).
What is the PKD Research Resource Consortium (RRC)?
Funded by NIDDK, the RRC’s goal is to advance PKD research by supporting a collaborative, diverse community of investigators. They provide important resources to help grow the study of PKD.
Unknown Causes of Kidney Disease Summit
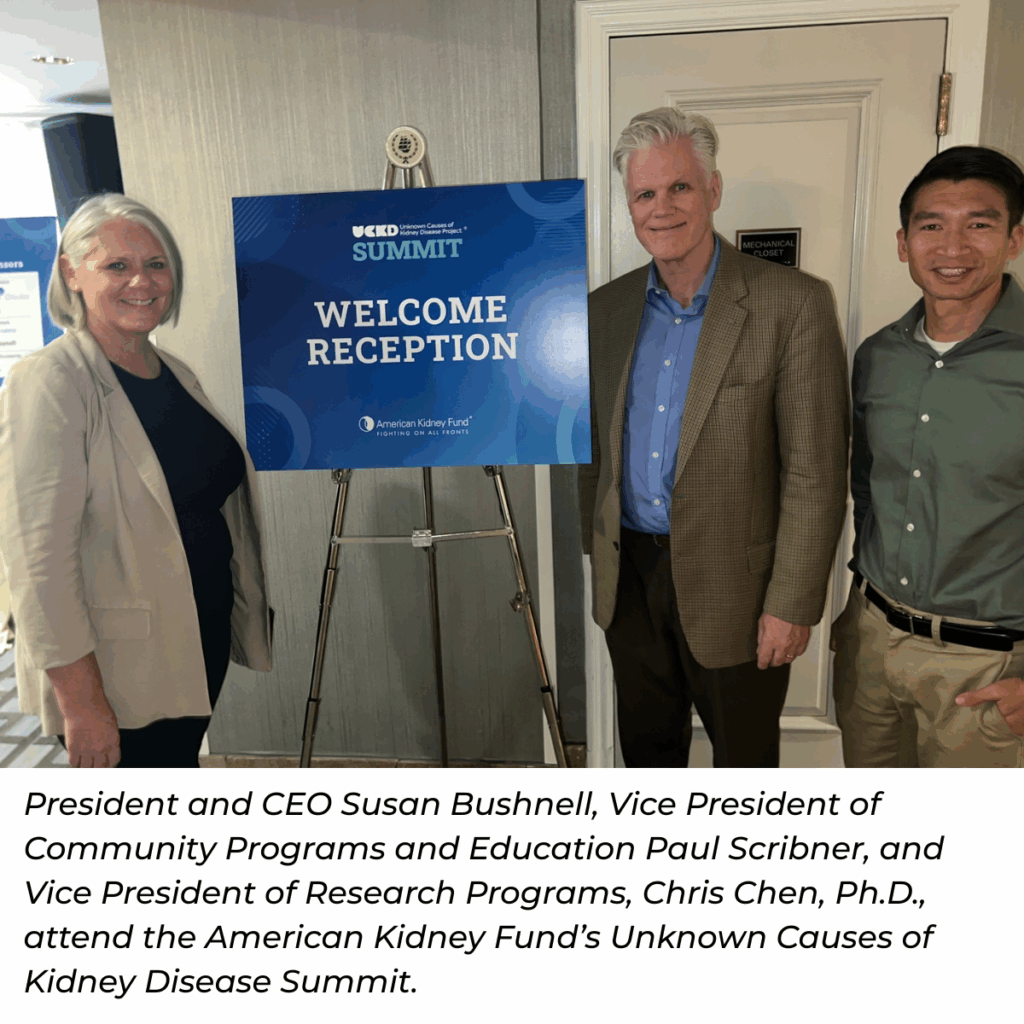
PKD Foundation leadership, including President and CEO Susan Bushnell, Vice President of Research Programs, Chris Chen, Ph.D., and Vice President of Community Programs and Education Paul Scribner, attended the American Kidney Fund’s (AKF) Unknown Causes of Kidney Disease Summit. The summit focused on the Unknown Causes of Kidney Disease (UCKD) Project. Launched in 2020, this initiative seeks to “improve understanding of how undiagnosed kidney disease or misdiagnosed causes of kidney disease directly impact patient care and outcomes.”
During the summit, sessions emphasized how rural outreach and multidisciplinary care are key in tackling undiagnosed kidney disease. This included topics such as education and genetic testing. Additionally, a panel of advocates shared their experiences, shining an important spotlight on patient voices.
Patient Perspectives in PKD Foundation Programs
At the PKD Foundation, the voices and perspectives of PKD patients are vital to our work. When selecting which grants and fellowships to fund, we ensure that the needs and perspectives of PKD patients are at the forefront. This includes having patient volunteers participate in the grant review process.
Through our recent survey, we captured feedback from patients, caregivers, clinicians, and others to understand the particular educational, social, emotional, financial, and other support needs of our community. This will help us develop initiatives and improve programs and services for the overall community.

What Does This Mean for the Future of PKD Research and Clinical Trials?
Summits, conferences, and scientific meetings are unique opportunities for collaboration and innovation. Through these events, our leadership can connect with leading experts, patients, pharmaceutical companies, and other kidney health organizations to grow PKD research and chart a path to new treatments and a cure.

“At the PKD Foundation, we know that progress doesn’t happen in isolation. Every conversation and collaboration helps accelerate PKD research and advance clinical trials. By uniting researchers, regulators, industry leaders, and patients, we’re building momentum to drive new treatments and a cure for PKD.”
—Susan Bushnell, PKD Foundation President and CEO
Partnerships power progress. Today, the PKD Foundation and PKDOC are actively working to advance PKD clinical trials. Our projects include:
- Developing new biomarkers for PKD clinical trials
- Creating tools and models that help researchers test potential treatments
- Partnering with patients, researchers, industry, and the FDA to design the next generation of ADPKD and ARPKD clinical trials
If you’re interested in PKD research or PKD clinical trials, you can:
- Sign up for ADPKD or ARPKD ACT Alerts. These emails let you know when a clinical trial is seeking participants.
- Listen to our podcast, PKD Chronicles. Episode four discusses TKV, and episode five dives into clinical trials.
- Join the ADPKD Registry. As a participant, you’ll be matched with ongoing clinical trials.